Introduction: Therapy-related myeloid neoplasms are associated with a previous exposure to cytotoxic agents or ionizing radiation, and account for 10-20% of all myeloid neoplasms. They are associated with a worse outcome, usually with high risk genetic abnormalities and poor response to therapies. Chronic myelomonocytic leukemia (CMML) is a rare myeloid neoplasm with persistent monocytosis, bone marrow dysplasia and an inherent risk of acute myeloid leukemia (AML) transformation. Due to its rarity, it is difficult to establish the specific impact of previous therapy in CMML. We previously reported the characteristics and outcomes of patients with therapy-related CMML (tCMML). Herein we report an update, including molecular characteristics, being the largest tCMML cohort reported to date.
Methods: We retrospective evaluated a cohort of patients diagnosed with CMML from 2005 to 2022. Data regarding previous exposure to treatment was captured and tCMML diagnosis was established for patients with a documented prior history of radiation therapy or cytotoxic chemotherapy due to an antecedent malignancy prior to the diagnosis of CMML. Those non-tCMML were defined as de novo CMML (dnCMML). Overall survival (OS) was calculated from diagnosis to death, and leukemia-free survival (LFS) was calculated from diagnosis to death or AML transformation.
Results: A total of 532 patients with CMML were included in the study, of whom 71 (13%) were tCMML. When comparing tCMML with dnCMML, there were no differences in median age (73 vs 70 years old) or sex (67% vs 69% male). No differences were noted in white blood cell count, absolute monocyte count, hemoglobin or platelet count. According to the WHO 2016 classification, 38% vs 44% were CMML-0, 40% vs 36% were CMML-1, and 22% vs 20% were CMML-2, for patients with dnCMML and tCMML, respectively. According to the FAB classification, 42% vs 52% were dysplastic and 58% vs 49% were proliferative, for patients with dnCMML and tCMML, respectively. Chromosome 7 abnormalities where more common among tCMML compared to dnCMML (14% vs 4%, p=0.006), with no differences in frequency of complex karyotype (8% vs 3%, p=0.1).
In tCMML patients, 35% received only radiotherapy, 31% only chemotherapy and 34% both. In patients that received chemotherapy, the most common therapies were alkylating agents (45%), agents targeting microtubules (37%) and antimetabolite agents (35%). The median latency from therapy to tCMML was 6.5 years (range, 0.1-24). Most common neoplasms prior to the diagnosis of tCMML were prostate (25%), lymphoma (23%) and breast cancer (14%).
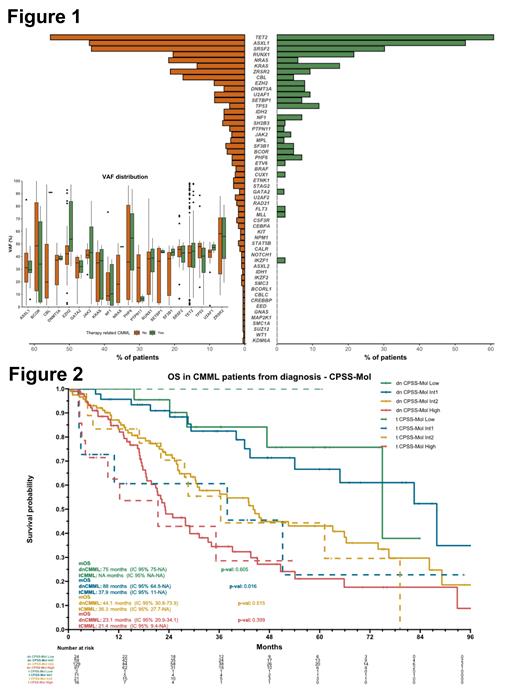
Most frequently detected mutations at diagnosis were TET2 (55% vs 61%, p=0.5), ASXL1 (44% vs 53%, p=0.3), and SRSF2 (43% vs 30%, p=0.1), in patients with dnCMML and tCMML, respectively. Patients with tCMML had a lower incidence of NRAS (22% vs 6%, p=0.007) and CBL (18% vs 5%, p=0.04) mutations compared to dnCMML, but these mutations were present at a higher median VAF among patients with tCMML (48% vs 18% [p=0.04] for NRAS and 91% vs 20% [p=0.02] for CBL) (Figure 1). TP53 mutations were more frequent in the tCMML although differences were not significant (12% vs 4%, p=0.06).
Among all patients, 352 patients received treatment and were evaluable for response (305 patients with dnCMML and 47 patients with tCMML). More than 90% of patients received an hypomethylating agent-based therapy, with an ORR of 59% for dnCMML and 60% for tCMML.
The median follow-up was 58 months, and the median OS and LFS for the entire cohort were 36 and 29 months, respectively. No differences in OS (36 vs 35 months, p=0.3) or LFS (29 vs 28 months, p=0.8) were observed between dnCMML and tCMML, However, when comparing by risk groups according to CPSS-Mol, patients with tCMML classified as intermediate-1 (Int-1) risk had a significant lower OS (38 vs 88 months, p=0.02) and LFS (38 vs 85 months, p=0.03), compared to Int-1 dnCMML (Figure 2). In a multivariate model for OS including CPSS-mol, tCMML stratified by type of therapy and age, patients with tCMML with a previous exposure to chemotherapy had a hazard ratio of 1.76 (1.07-2.89, p=0.03).
Conclusion: Patients with tCMML have a higher proportion of chromosome 7 abnormalities and a lower incidence of NRAS and CBL mutations. Although responses to HMA were similar, patients with tCMML with a previous exposure to chemotherapy are associated with a lower survival, especially patients classified as CPSS-Mol intermediate-1.
Disclosures
Chien:Rigel Pharmaceuticals: Consultancy; AbbVie: Consultancy. DiNardo:Fogham: Honoraria; ImmuniOnc: Honoraria; AbbVie/Genentech: Honoraria; Astellas: Honoraria; BMS: Honoraria; Notable Labs: Honoraria; Servier: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Schrödinger: Consultancy. Ravandi:Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Amgen: Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees. Kadia:Amgen, Inc.: Research Funding; Ascentage Pharma Group: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Genzyme: Honoraria; Astellas Pharma Global Development: Research Funding; Cellenkos Inc.: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; AstraZeneca: Research Funding; Cyclacel: Research Funding; Celgene: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Cure: Speakers Bureau; GenFleet Therapeutics: Research Funding; Glycomimetics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Iterion: Research Funding; Janssen Research and Development: Research Funding; Liberum: Consultancy; Novartis: Consultancy; Pulmotect, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Regeneron Pharmaceuticals: Research Funding; Sanofi-Aventis: Consultancy; SELLAS Life Sciences Group: Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy; Agios: Consultancy; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Kantarjian:Pfizer: Honoraria; Novartis: Honoraria; KAHR Medical: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Immunogen (Inst): Honoraria, Research Funding; Daiichih-Sankyo (Inst): Honoraria, Research Funding; AstraZeneca/MedImmune: Honoraria; Astellas Pharma: Honoraria; Ascentage Pharma Group: Honoraria; Amgen: Honoraria; Abbvie: Consultancy, Honoraria; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Taiho Pharmaceutical: Honoraria; Abbvie (Inst): Research Funding; Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Novartis (Inst): Research Funding. Garcia-Manero:Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding; AbbVie: Research Funding. Montalban-Bravo:Rigel: Research Funding; Takeda: Research Funding.